The many colors of hydrogen
 I had been following the development of a “hydrogen economy” out of the corner of my eye, but I was caught flat-footed when a friend asked me: “What’s the difference between green and blue hydrogen?”
I had been following the development of a “hydrogen economy” out of the corner of my eye, but I was caught flat-footed when a friend asked me: “What’s the difference between green and blue hydrogen?”
I didn’t know it came in colors. So, I dug into this question and found out that hydrogen does indeed come in “colors.” And not just two.
Hydrogen is the lightest and most abundant element in the universe and one of the main ingredients of organic molecules and water. Particularly over the past couple of decades, it has been considered a possible fuel.
It is important to note that hydrogen is not a source of energy, but a conveyor of energy – just like the electricity that comes into your house. In both cases, there is a primary energy source where the energy or fuel originates and is converted to another form, transported and converted again for use.
Fuel characteristics
As a fuel, hydrogen has several appealing characteristics. It has a high energy to mass ratio. Two pounds of hydrogen has about the same energy content as six pounds of gasoline. Initially, hydrogen was considered for use as a substitute for gasoline. However, it burns very hot and produces greenhouse gases when mixed with air for combustion.
Another way to transform hydrogen into a useful form of energy is a fuel cell. This technology has improved greatly over the past decade or so and is now the leading choice for transportation applications. Fuel cells are like batteries that never need charging. You just need a source of hydrogen, and it is totally clean when used in a fuel cell. Water is the only byproduct. And don’t forget, there is virtually an infinite supply of this stuff.
As with any fuel, one has to deal with storage, transport and production. All three present challenges, but production is the most dominant. Any production method has to be cost-competitive, operate on a large scale and be environmentally clean.
Producing hydrogen
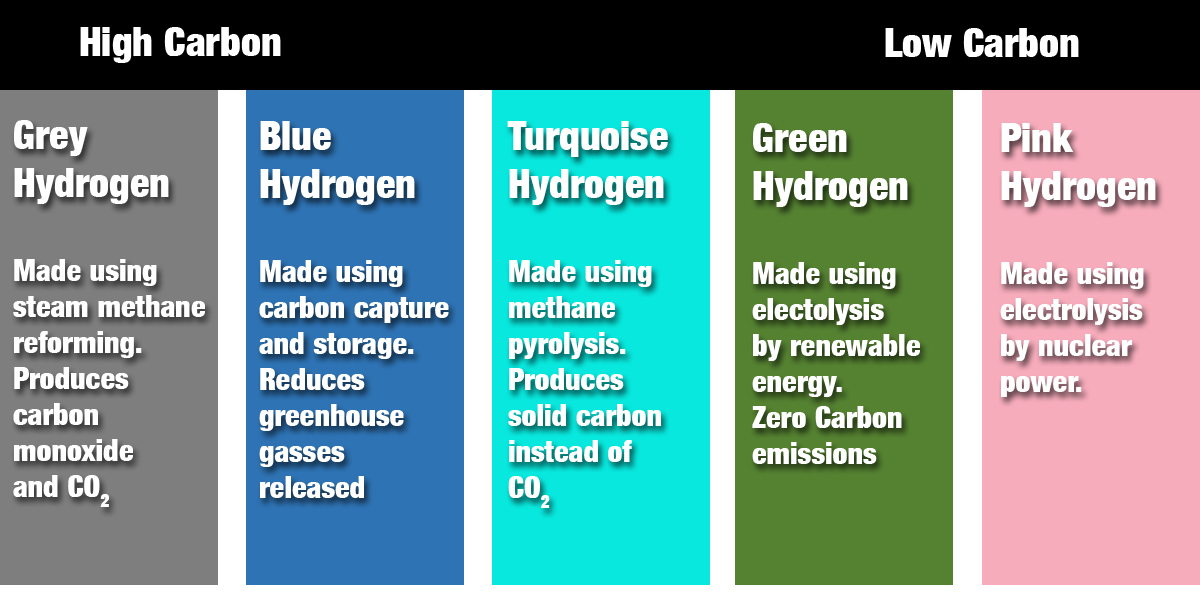
Very little natural hydrogen is found around the world, so it needs to be separated from other molecules. There are many methods to achieve this, and they are identified by assigning a “color.”
The most common large-scale process is steam methane reforming (SMR), which is used to produce hydrogen for making ammonia fertilizer and in hydrocracking to break down heavy petroleum for fuel.
However, it also produces carbon monoxide and CO2. Hydrogen produced by this method is called “gray” hydrogen.
A process called carbon capture and storage (CCS) can reduce the amount of greenhouse gas released. Hydrogen produced by this method is “blue” hydrogen.
A fairly recent method called methane pyrolysis only produces solid carbon but has not been developed for large scale use yet. This is how we get “turquoise” hydrogen.
Electrolysis
One more general production method is to split water into hydrogen and oxygen. There are several ways to do this, but the most common is by electrolysis – where an electric current is passed between two electrodes.
Renewable energy sources such as wind and solar can provide the electricity to produce “green” hydrogen. Electrolysis can also be achieved using nuclear power. Then we get “pink” hydrogen.
There is a lot more to the hydrogen story than I can go into here. It’s a hot topic, and government and industry are investing heavily in development. I think I’ll keep a closer eye on this in the future.
Our own Berkeley Lab is partnering with other national laboratories to develop the technology to enable the use of hydrogen. For more info, check out moving-hydrogen-technologies-forward or hydrogen.energy.gov. Read more Science Bytes columns here.

Steve Gourlay
Steve Gourlay is a career scientist with a PhD in experimental particle physics. He recently retired after working at the Fermi National Accelerator Laboratory, CERN (the European Center for Nuclear Research) and the Lawrence Berkeley National Laboratory. Send questions and comments to him at sgpntz@outlook.com.
