Probing the secrets of the early days of matter
 In my experience, the simplest questions are often the most difficult to answer. I’ve been stumped many times by 4-year-olds.
In my experience, the simplest questions are often the most difficult to answer. I’ve been stumped many times by 4-year-olds.
This month, I’ll tackle a question that has been asked for millennia by young and old: “What is the universe made of?”
Getting to that answer depends on what tools we have available. We can use our eyes to determine the color and shape of an object, and we can measure mechanical and electrical properties. But how do we see inside?
As an example, imagine a sealed cardboard box. We can determine the mass and measure the size. But how is the mass distributed inside the box? What objects does it contain?
One thing we can do is fire projectiles at it and see what happens. Do they go straight through or bounce off something in the box?
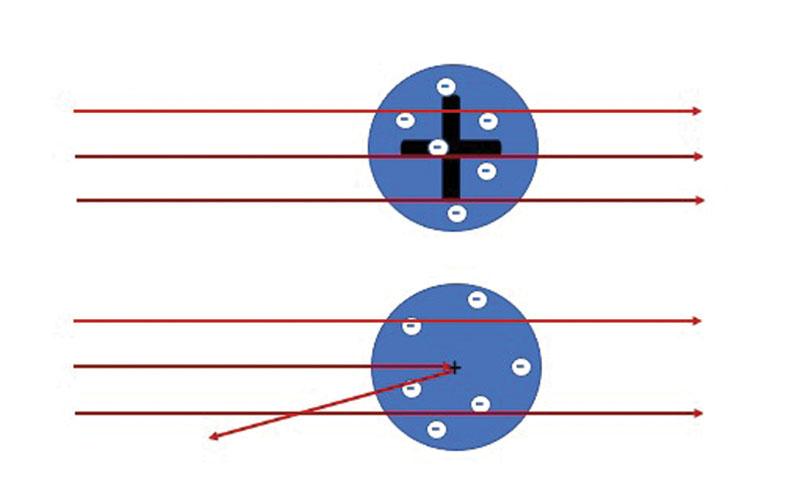
At the beginning of the 20th century, we knew about the existence of atoms but the actual structure was a heavily debated topic. British physicist J.J. Thompson had recently discovered the first subatomic particle, the electron. He proposed the so-called “plum pudding” model of the atom as a volume of positive charge with negatively charged electrons embedded like plums or raisins in a pudding. This is what Thompson assumed to be in “the box.”
However, for several reasons, this model was not universally accepted. It was a hypothesis that needed to be tested.
Under the direction of Ernest Rutherford, a New Zealand-born physicist, researchers performed a series of experiments at the University of Manchester from 1908 to 1913. The scientists, including Hans Geiger and Ernest Marsden, used a natural radioactive source of alpha particles (helium nuclei) as projectiles to strike a thin gold target. Rutherford analyzed the experimental data and found a surprising result.
“It was quite the most incredible event that has ever happened to me in my life,” he said later. “It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
According to the plum pudding model, the alpha particles should have passed straight through the foil. Based on this strange result, the picture of an atom was of a very small, positively charged “nucleus” containing most of the mass of the atom with the electrons outside.
This implied that atoms were mostly empty space.
To get a feel for this, imagine that we enlarge the proton in a hydrogen atom to the size of a ping pong ball. Then the electron would be, on average, about half a mile away.
This discovery opened a whole other can of worms. Why were atoms stable? Wouldn’t the electron and proton attract each other and collapse the atom? This led to the invention of quantum mechanics and special relativity.
Quantum mechanics tells us that light can behave like a particle and particles can behave like waves and that the wavelength of a particle is inversely proportional to its momentum.
So particles with greater momentum have a shorter wavelength and allow us to probe smaller distances or, as in our example, look inside smaller boxes. This takes us into the realm of particle accelerators, the primary tools for studying the subatomic world.

Steve Gourlay
Steve Gourlay is a career scientist with a PhD in experimental particle physics. He recently retired after working at the Fermi National Accelerator Laboratory, CERN (the European Center for Nuclear Research) and the Lawrence Berkeley National Laboratory. Send questions and comments to him at sgpntz@outlook.com.
